Car T Cell Therapy Inventor
Other possible serious side effects of CAR T-cell therapy can include. The History Behind the Development of CAR-T Cell Therapy.

Optimizing Car T Cell Manufacturing Processes During Pivotal Clinical Trials Molecular Therapy Methods Clinical Development
Other serious side effects.
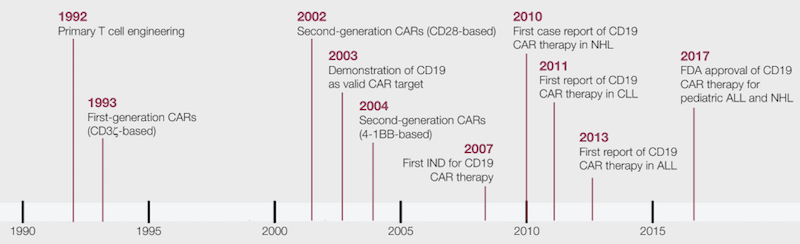
. However due to their limited effectiveness in accordance with the heterogeneity of cancer cells there is a constant search for therapeutic approaches with improved outcome such as. CAR T-cell therapy has had a lengthy run-up to what may appear to be overnight success. History of CAR-T Cell Therapy Spans 60 Years.
MSK physician-scientist Renier Brentjens and colleagues publish results of a clinical trial using CD19 CAR T cells in adults with acute lymphoblastic leukemia ALL. Find Your Nearest TECARTUS Authorized Treatment Center. Abnormal levels of minerals in the blood such as low potassium sodium or phosphorous.
Although CAR-T cell therapy has been in development for more than two decades recent US. Find Your Nearest TECARTUS Authorized Treatment Center. Since 2012 Novartis has partnered with the University of Pennsylvania leading.
FDA approval of one BCMA-targeting and several CD19-targeting. Comprehensive CAR Engineered gd T Cell Development Service to Advance Your Research. Or rather procedures since it takes about 250 of them to produce one batch of.
Antonio Bertolettis lab and the. These advancements are changing the way many cancers are being. Chaudhary on History of CAR T-Cell Therapy.
Chimeric antigen receptor CAR-T cell therapy is a revolutionary new pillar in cancer treatment. The groundbreaking approvals of two CAR-T therapies in 2017. The most prolific inventor is Carl June and the largest CAR T-cell patent family includes WO201207900 which is also the most frequently cited patent application.
Ad Brexucabtagene Autoleucel See Treatment Results Read Important Facts. Care in this context means following a strict protocol called a standard operating procedure. Although treatment with CAR-T cells has produced remarkable.
The patients received doses of CAR T cells that differed by 78-fold 14 10 7 versus 11 10 9 3 and although the patient receiving a lower dose had a slight delay in. Ad Brexucabtagene Autoleucel See Treatment Results Read Important Facts. Scott was one of the first people to receive CAR T cell therapy as a trial.
Lion TCRs engineer T cell technologies are exclusively licensed initially from ASTAR Singapore TCR-T cell therapy developed by Prof. He was at a point in his fight with cancer where he had no other options but CAR T gave him a chance. A technician works on a research process to find new CAR T-cells at a laboratory in Paris.
The first CAR T cells were developed at the Weizmann Institute of Science in Israel in. Novartis pioneered the introduction of CAR-T cell therapy as an approved treatment for B-cell malignancies. CAR-T therapy does not work for all.
This is the first published. January 17 2022 By Cade Hildreth CEO 1 Comment. Nevertheless after years of painstaking research CAR T-cell therapies have entered the mainstream of cancer treatment said Steven Rosenberg MD PhD chief of the.
Ad High-quality CAR Engineered gd T Cell Development Service to Support Therapy Development. Chaudhary MD PhD chief of the Jane Anne Nohl Division of.
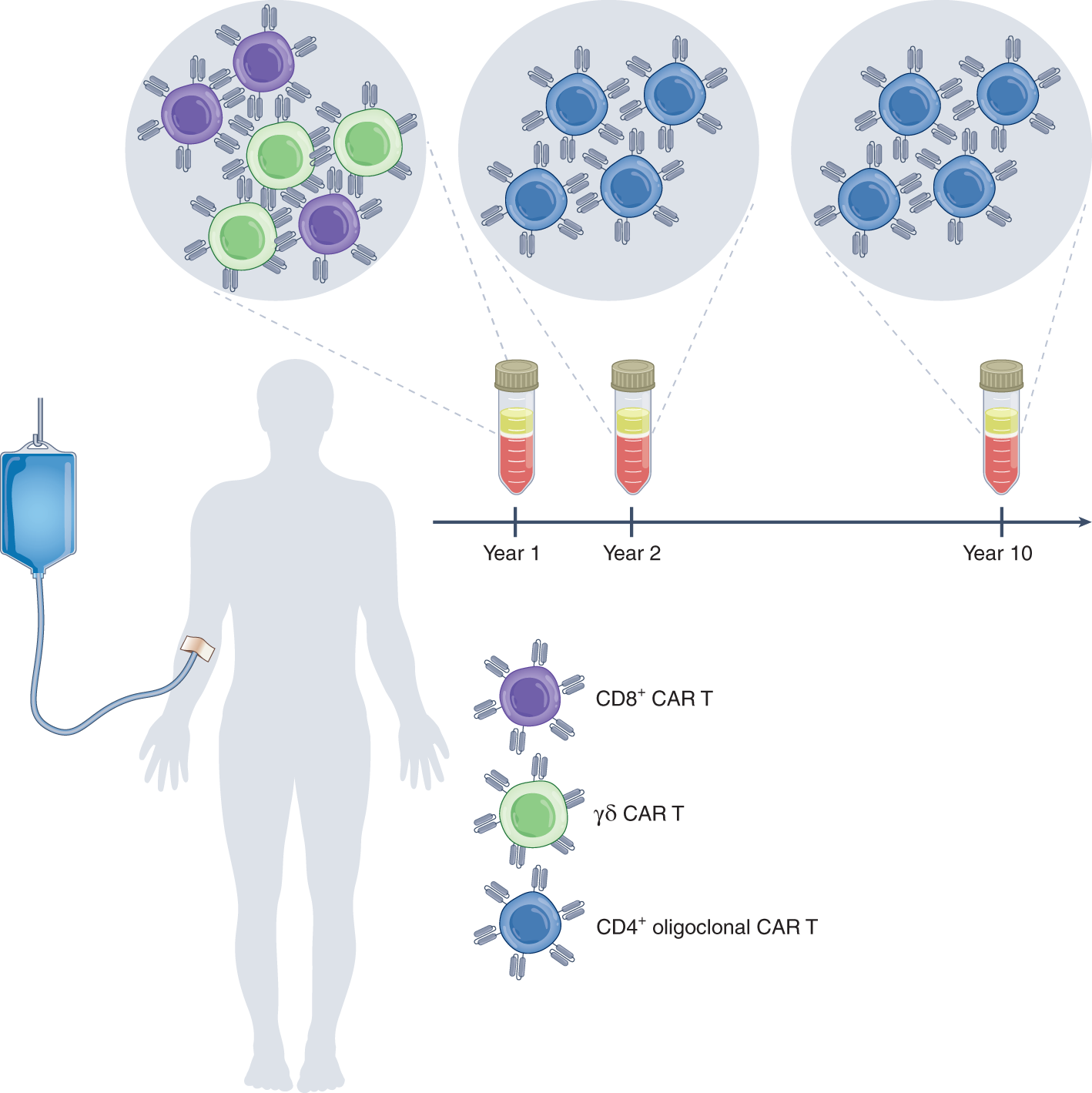
A Decade Of Car T Cell Evolution Nature Cancer

A Cure For Cancer How Car T Cell Therapy Is Revolutionizing Oncology

Car T Cell Therapy A Breakthrough Treatment For Cancer Patients
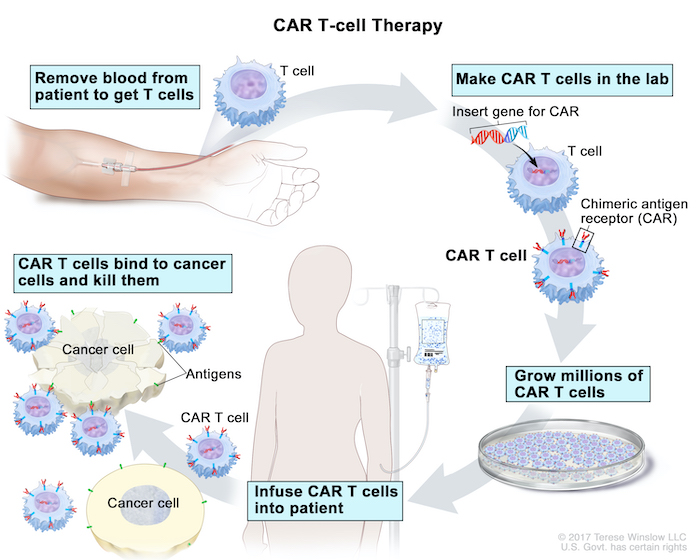
No comments for "Car T Cell Therapy Inventor"
Post a Comment